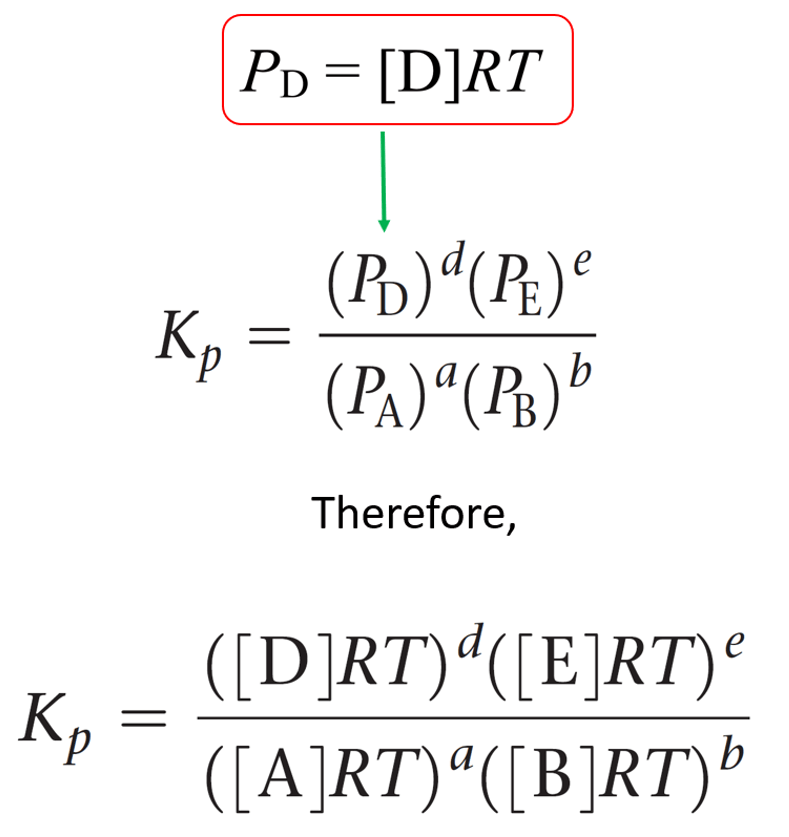Calculate K Equation . In this video, we'll calculate equilibrium constants using measurements of. Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). Calculating the equilibrium constant from equilibrium concentrations. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. The key to solving this. Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Calculate equilibrium concentrations from initial concentrations. Enter the coefficients and concentrations in their designated fields.
from general.chemistrysteps.com
Calculate equilibrium concentrations from initial concentrations. In this video, we'll calculate equilibrium constants using measurements of. Enter the coefficients and concentrations in their designated fields. Calculating the equilibrium constant from equilibrium concentrations. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. The key to solving this.
Kp Equilibrium Constant and Partial Pressure Chemistry Steps
Calculate K Equation Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). In this video, we'll calculate equilibrium constants using measurements of. Calculating the equilibrium constant from equilibrium concentrations. Calculate equilibrium concentrations from initial concentrations. Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Enter the coefficients and concentrations in their designated fields. The key to solving this. Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical.
From www.youtube.com
Calculating K from Concentration YouTube Calculate K Equation Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Calculate equilibrium concentrations from initial concentrations. The key to solving this. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. In this section,. Calculate K Equation.
From blog.sepscience.com
Back to Basics 10 Fundamental Resolution Equation k Calculate K Equation Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. Calculating the equilibrium constant from equilibrium concentrations. In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). In this video, we'll calculate equilibrium constants using measurements of. The key to solving this. Calculate. Calculate K Equation.
From www.slideserve.com
PPT The Equilibrium Constant, K, and The Reaction Quotient, Q Calculate K Equation The key to solving this. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. Calculate equilibrium concentrations from initial concentrations. In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). Enter the coefficients and concentrations in their designated fields. In this video,. Calculate K Equation.
From www.youtube.com
Equilibrium Constant from Concentration (Example) YouTube Calculate K Equation In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Calculate equilibrium concentrations from initial concentrations. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. In this. Calculate K Equation.
From www.slideserve.com
PPT Chapter 6 Turbulence Modeling PowerPoint Presentation, free Calculate K Equation Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Enter the coefficients and concentrations in their designated fields. Calculate equilibrium concentrations from initial concentrations. Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Calculating the equilibrium constant from equilibrium concentrations. In this section, we explore the relationship between. Calculate K Equation.
From haipernews.com
How To Calculate Equilibrium Constant K Haiper Calculate K Equation Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. Enter the coefficients and concentrations in their designated fields. Calculating the equilibrium constant from. Calculate K Equation.
From feevalue.com
find the value of k so that the quadratic equation If the equation (k+1 Calculate K Equation In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). Calculate equilibrium concentrations from initial concentrations. Calculating the equilibrium constant from equilibrium concentrations. Enter the coefficients and concentrations in their designated fields. In this video, we'll calculate equilibrium constants using measurements of. Calculate \(k\) for the overall equation by. Calculate K Equation.
From www.youtube.com
53 (k3)x+3y=k kx+ky=12 for each of the following systems of Calculate K Equation In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). Enter the coefficients and concentrations in their designated fields. In this video, we'll calculate equilibrium constants using measurements of. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. Calculate \(k\) for the. Calculate K Equation.
From www.youtube.com
Example Kp calculations YouTube Calculate K Equation Calculating the equilibrium constant from equilibrium concentrations. Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). Enter the coefficients and concentrations in their designated fields. Calculate the product of equilibrium concentrations of reactants. Calculate K Equation.
From www.tessshebaylo.com
How To Find The Value Of K In A Quadratic Equation With Equal Roots Calculate K Equation Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. In this video, we'll calculate equilibrium constants using measurements of. The key to solving this. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced. Calculate K Equation.
From oneclass.com
OneClass Complete the equations for the following equilibria and Calculate K Equation The key to solving this. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). In this video, we'll calculate equilibrium constants using measurements of. Calculate equilibrium concentrations from initial concentrations. Calculating the. Calculate K Equation.
From www.youtube.com
Calculate k Using Data YouTube Calculate K Equation Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Calculate equilibrium concentrations from initial concentrations. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. Calculating the equilibrium constant from equilibrium concentrations. In this section, we explore the relationship between the free energy change of reaction (δg) and. Calculate K Equation.
From sciencenotes.org
pH, pKa, Ka, pKb, and Kb in Chemistry Calculate K Equation Enter the coefficients and concentrations in their designated fields. Calculating the equilibrium constant from equilibrium concentrations. The key to solving this. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Calculate \(k\) for the overall equation by multiplying. Calculate K Equation.
From www.numerade.com
SOLVED The pH of a solution depends upon the concentration of the acid Calculate K Equation Calculating the equilibrium constant from equilibrium concentrations. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. The key to solving this. Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Enter the coefficients and concentrations in their designated fields. In this video, we'll calculate equilibrium constants using. Calculate K Equation.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc YouTube Calculate K Equation In this video, we'll calculate equilibrium constants using measurements of. In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. Calculating the equilibrium constant from equilibrium concentrations. Calculate \(k\) for the overall equation. Calculate K Equation.
From printablecampusholzman.z21.web.core.windows.net
List Of Equilibrium Constants Calculate K Equation In this video, we'll calculate equilibrium constants using measurements of. Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Calculate equilibrium concentrations from initial concentrations. In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). The key to solving this. Calculating the equilibrium. Calculate K Equation.
From www.chegg.com
Solved Determine the value of k such that the linear system Calculate K Equation The key to solving this. Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Enter the coefficients and concentrations in their designated fields. In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). Calculate the product of equilibrium concentrations of reactants (raised to. Calculate K Equation.
From www.tessshebaylo.com
For Which Value Of K Will The Following Pair Linear Equations Have No Calculate K Equation In this video, we'll calculate equilibrium constants using measurements of. Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Calculating the equilibrium constant from equilibrium concentrations. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. Enter the coefficients and concentrations in their designated fields. The key to. Calculate K Equation.